ClinOleic
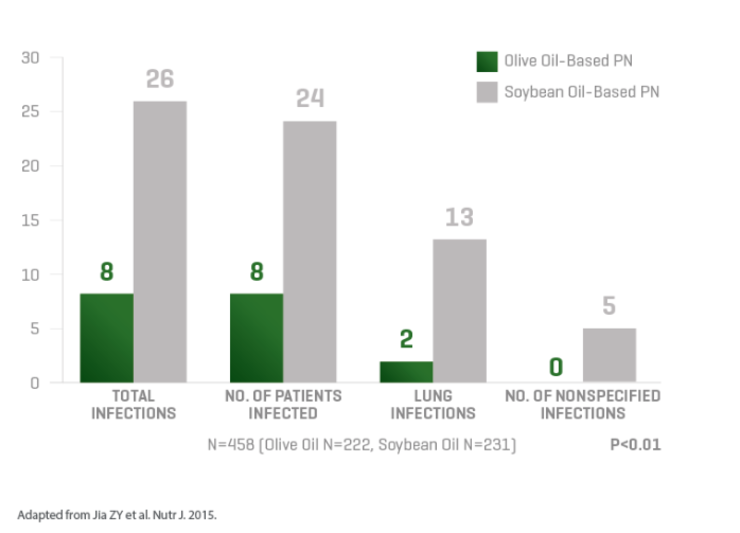
An olive oil-based lipid emulsion that is associated with fewer infections than soybean oil and may preserve immune function.1-4

Lipids are an integral part of parenteral nutrition (PN), but some lipid emulsions, such as 100% soybean oil, may modulate patient immune response and increase the risk of complications.3,5 Through 20 years of experience, ClinOleic maintains the lowest soybean oil content of any soy-containing composite lipid emulsion.6-11 ClinOleic also contains 80% olive oil, which has been associated with fewer infections and may preserve immune function.1-4

Aligned with international guidelines
ASPEN guidelines recommend to withhold or limit soybean oil-based lipid emulsions during the first week following the initiation of PN.18 Additionally, ESPEN guidelines recommend that lipids based solely on soybean oil be avoided.14 ClinOleic has the lowest soybean oil content of any soy-containing composite lipid product on the market, enabling the delivery of essential fatty acids while minimizing the unwanted effects of soybean oil.6-11,15
Learn more about Clinical Nutrition

Related Products
FACHKURZINFORMATION
Clinoleic 20 % - Emulsion zur Infusion
ATC - Code: B05BA02
QUALITATIVE UND QUANTITATIVE ZUSAMMENSETZUNG: 100 ml enthalten: Gereinigtes Olivenöl und gereinigtes Sojaöl* 20,00 g entsprechend einem Gehalt an essentiellen Fettsäuren von 4,00 g * Mischung aus gereinigtem Olivenöl (ca. 80 %) und gereinigtem Sojaöl (ca. 20%) Energiegehalt 2000 kcal/l (8,36 MJ/l) Fettgehalt (Oliven- und Sojaöl) 200 g/l Osmolarität 270 mOsm/l pH-Wert 6-8 Dichte 0,986 Phospholipide, entsprechend 47 mg oder 1,5 mmol Phosphor pro 100 ml Liste der sonstigen Bestandteile: Phospholipide aus Eiern, Glycerol, Natriumoleat, Natriumhydroxid, Wasser für Injektionszwecke
ANWENDUNGSGEBIETE:
Indiziert zur Fettzufuhr für Patienten, die parenteral ernährt werden müssen, wenn eine orale oder enterale Ernährung unmöglich, unzureichend oder kontraindiziert ist.
GEGENANZEIGEN:
Clinoleic 20 % ist in folgenden Situationen kontraindiziert: Überempfindlichkeit gegen Ei-, Soja- oder Erdnussprotein oder gegen einen der Wirkstoffe oder sonstigen Bestandteile, schwere Dyslipidämie und nicht korrigierte Stoffwechselstörungen wie Laktatazidose und entgleister Diabetes.
INHABER DER ZULASSUNG:
Baxter Healthcare GmbH, Stella-Klein-Löw-Weg 15, 1020 Wien
STAND DER INFORMATION
VERSCHREIBUNGSPFLICHT/APOTHEKENPFLICHT: Rezept- und apothekenpflichtig.
Weitere Angaben zu „Besondere Warnhinweise und Vorsichtsmaßnahmen für die Anwendung“, „Wechselwirkungen mit anderen Arzneimitteln und sonstige Wechselwirkungen“, „Fertilität, Schwangerschaft und Stillzeit“ und „Nebenwirkungen“ sind der veröffentlichten Fachinformation zu entnehmen.