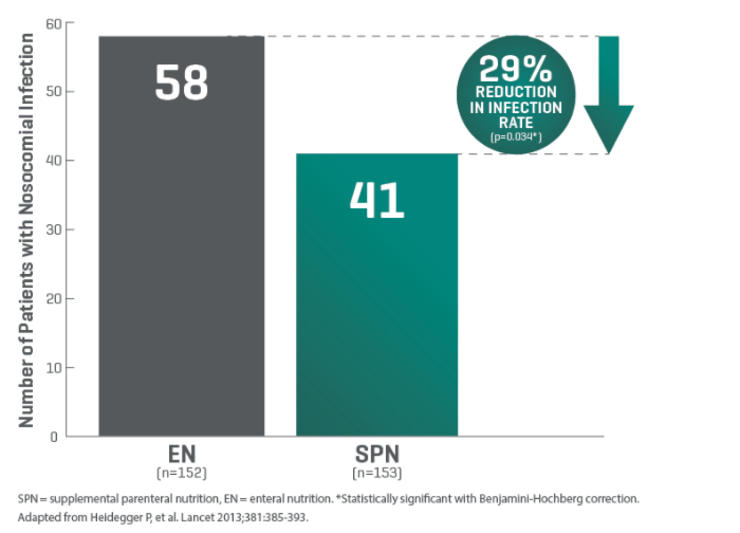
For critically ill patients who require nutritional support, Q-NRG+ offers quick and accurate resting energy expenditure (REE) measurement through indirect calorimetry (IC). International guidelines recommend use of indirect calorimetry to guide energy prescription to avoid under- and over- feeding.1,2
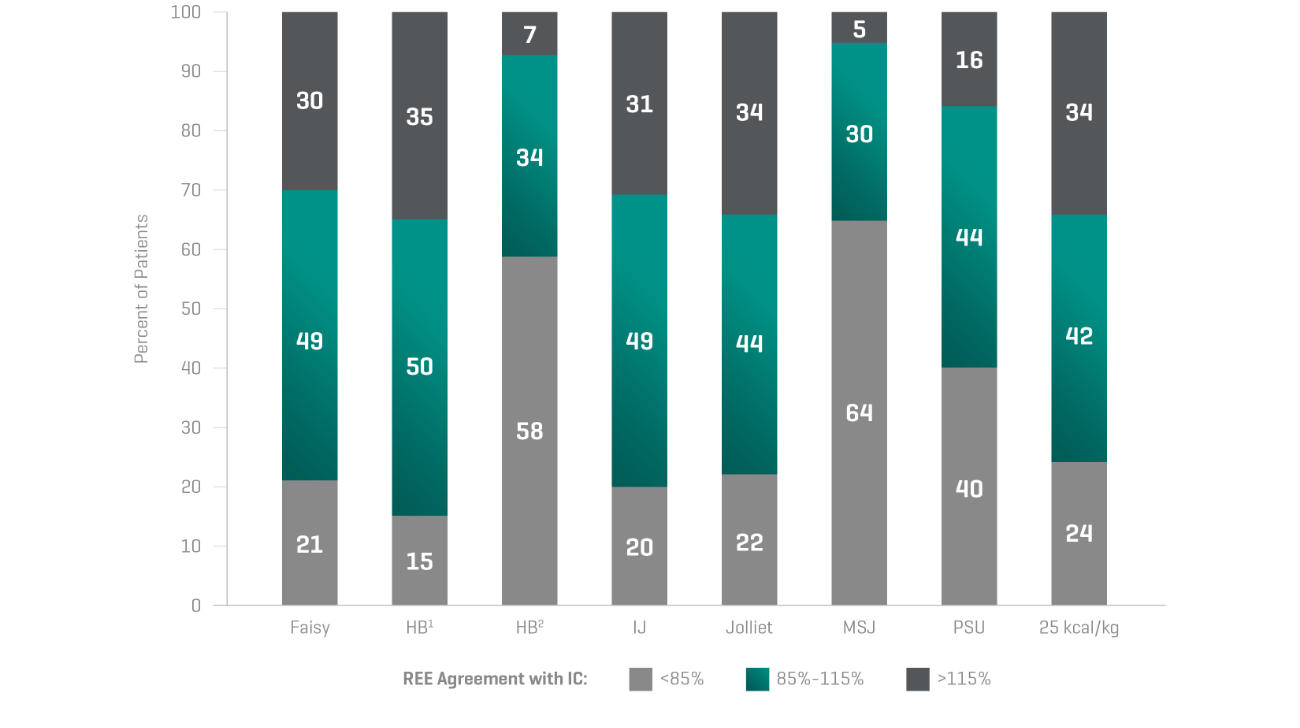
“The accuracy of predictive equations is very low.
And this could lead to overfeeding or underfeeding
the patient leading to increased mortality or morbidity.”Dr. Pierre Singer
Inaccuracy of predictive equations4
Fast and reliable measurements
Accurate measurement of resting energy expenditure in around 10-15 minutes up to 70% FiO2.
Portable and easy to use
User-friendly touch screen and battery-powered for easy transport in the hospital.
Seamless integration
Compatible with current nutrition therapy workflows and all ventilators, patient canopies or masks.
Minimal maintenance
Automatic daily and monthly calibrations for simple maintenance and operation.